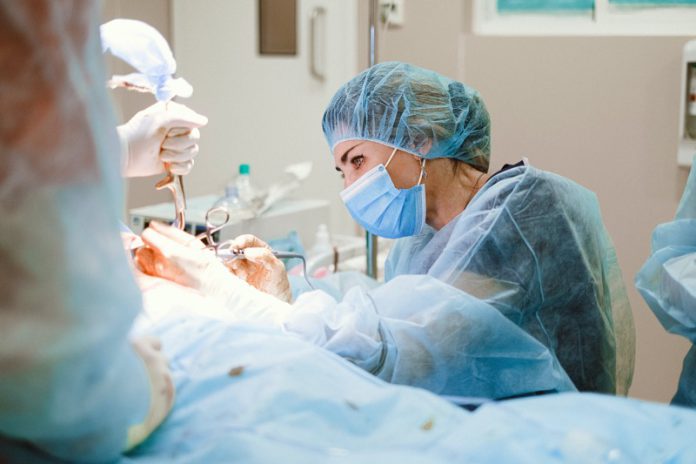
From fixing broken bones to replacing worn-out joints, medical devices play a crucial role in modern healthcare.
With the industry experiencing exponential growth, understanding the most popular materials enabling precise diagnoses and life-saving treatments is key. These materials allow incredible miniaturization and increase the reliability of products like pacemakers and glucose monitors.
However, issues can also arise when substandard materials are used or the materials degrade over time, thus, necessitating careful testing and monitoring. In this article, we will discuss the seven most commonly used materials in medical device manufacturing, their advantages and limitations, and their impact on patient safety.
Metals
Metal alloys are one of the most commonly used components in medical device manufacturing. Think of them as the skeleton frame behind those remarkable medical devices. They offer excellent strength, durability, and biocompatibility, making them ideal for multiple applications.
Examples of medical devices made from metal alloys include orthopedic implants, pacemakers, and dental implants. Titanium stands out for its exceptional strength and biocompatibility, which makes it suitable for dental implants, bone fixation devices, and joint replacements. Stainless steel, on the other hand, is known for its permanence and corrosion resistivity. It is used extensively in surgical instruments and permanent implants.
However, no metal is perfect—stresses may enable corrosion over time, and accumulated wear debris can prompt inflammatory reactions, necessitating implant replacement.
Companies must sufficiently test candidate metals for a given application early on to ensure adequate biocompatibility, hardness, and endurance to elongation/cracking over projected lifetimes. Unexpected issues have arisen in the past with hip implants shedding metallic debris through wear, causing device early failures.
According to TorHoerman Law, there have been concerns about the safety of Exactech hip, knee, and ankle implants. The Exatech lawsuit alleges that certain Exactech implants have caused serious injuries such as bone loss and degradation, swelling, and chronic joint pain.
Polymers and Plastics
Polymers and plastics offer unparalleled versatility as they can mimic human tissues, bend like rubber, and withstand heat. Thus, they are used in various medical devices, including catheters, sutures, syringes, and implantable devices.
Polymers like polyethylene and polypropylene boast customizable properties, catering to a diverse range of medical device needs. These materials find application in prosthetics, implants, and disposable medical items due to their adaptability and biocompatibility.
Similarly, plastics, known for their lightweight nature and elasticity, are used in packaging, disposable medical products, and specific implantable devices. Polyether ether ketone (PEEK), a superpowered plastic, is used in spinal implants, thanks to its strength, high heat resistance, and diagnostic imaging compatibility.
However, there are also safety considerations associated with the use of polymers and plastics in medical devices. The risks include the leaking of chemicals into the body, accelerated component aging and brittleness due to heat, and the potential for bacterial growth.
It’s important to highlight certain products, like the Bard Power Port. This medical device, commonly used for intravenous access, is facing scrutiny for fracturing, leaks, and catheter breaks requiring surgical removal procedures. This resulted in a Bard Power Port lawsuit, which claims that certain Bard Power Port catheters can cause serious injuries and health complications.
Ceramics
Ceramics like zirconia and alumina find increasing adoption in bone screws, hip, knee, and dental replacements due to durability, biocompatibility, high corrosion resistance, and bone-bonding tendencies.
However, hardness and brittleness tradeoffs still apply. There’s always a risk of micro-cracks due to routine load impacts or sudden trauma, which leads to fractures if not detected early. Replacement challenges also exist—should ceramic components crack years later, juxtaposed bone and tissue may hinder modular component replacements.
Composites
Composites are materials made from two or more different substances, such as polymers and ceramics. They offer a combination of properties that cannot be achieved with a single material, such as high strength and low weight.
Composites also retain polymer benefits like flexibility and bio-stability, while enhancing wear resistance and bone in-growth potential from added particles. They are used in various medical devices, including prosthetics, implants, orthotics, and surgical instruments.
However, composites aren’t without faults. The same implant design can display drastically different characteristics depending on how it’s manufactured. Minor differences in mixing the components can lead to drastic changes in flexibility, wear, and bone growth.
Manufacturers need strict quality testing to catch issues early. Or else it will be an issue down the road which may further need replacement surgeries.
Biomaterials
Biological materials, such as tissues, absorbable sutures, breast implants, and artificial organs, are used in place of damaged tissues or organs. They can also be used to heal some damaged body parts.
Biomaterials play a crucial role in various medical applications, from tissue engineering to implantable devices, contributing to advancements in regenerative medicine and personalized healthcare solutions. However, there are also challenges of biological materials, such as the risk of infection, rejection, and wear and tear.
Paper and Other Fibers
While less common in medical device manufacturing, paper, and certain fibers find niche applications in specific medical contexts.
For instance, sterilized paper is utilized in packaging and as a disposable barrier in medical settings due to its affordability and ease of disposal. Similarly, cotton and silk are used in biocompatible suture thread materials. However, any variation in the natural raw goods can produce inconsistencies.
Moreover, organic fibers risk quicker biodegradation when compared to stable, long-lasting synthetic fibers. Yet, some improved natural fibers are eco-friendly choices that work well for medical devices meant for short-term use.
In conclusion, the materials used in medical device manufacturing are diverse and essential for the functionality and safety of these devices.
Metals, polymers and plastics, ceramics, composites, and biological materials each offer unique advantages and face specific limitations. Medical devices have come a long way, and the future looks even brighter.
With continuous research and responsible manufacturing, newer and better materials will further advance healthcare outcomes while minimizing risks. However, reasonable safety vigilance from regulators, manufacturers, and patients remains vital—though rare, some issues only arise years after device approvals, underscoring that safety is an enduring process, not a one-time event.